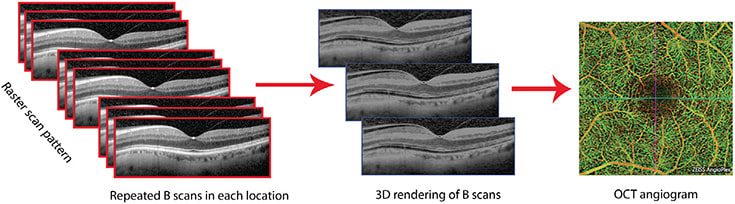
It has been two years since the introduction of OCT angiography (OCTA) to the ophthalmic landscape, introducing new and innovative ways to view retinal and choroidal pathology. OCTA is a revolutionary imaging modality that allows one to visualize vascular flow in the retina and choroid by utilizing motion-contrast en face OCT. En face, which means “on the face,” is a scanning technique that images the Z axis, or coronal plane, from anterior (vitreous) to posterior (sclera). This technique produces multiple scans from the tissue in between.1
OCTA is rapidly becoming a clinical standard for imaging of retinal pathology; most especially vascular disease. Although not at the point of replacing standard dye angiography for most disease identification, OCTA can be used as a stand-alone for initial diagnosis and for following treatment.
How it works
OCTA repeatedly scans an area with standard OCT B-scans and compares any change between scans from the same area using the volumetric data (Figure 1). Reflection or scattering from stationary tissue is constant, whereas OCT signals from moving tissue changes over time. In the posterior pole, blood flow is essentially the only moving structure. Pixels that show change are displayed as white, and pixels that are unchanged are displayed as black. OCTA does not require the injection of a dye, as standard fluorescein angiography does, but produces an image that closely mimics a fluorescein angiogram of the retinal vasculature (Figure 2).
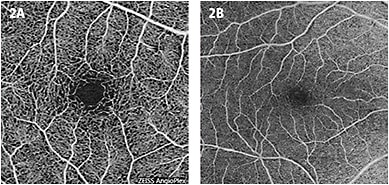
OCTA is built from three-dimensional OCT B-scan images but differs in the information it provides. Put simply, OCT displays anatomical structure, while OCTA displays vascular structure.
OCTA should not be considered a replacement for fluorescein angiography (FA), it is an adjunct imaging tool. With FA, one can visualize vascular permeability and the effects. Unless there is active vascular flow, disease pathology, such as RPE detachment and cystoid edema, will not be apparent on OCTA. However, since OCTA discards non-moving structures, such as pooling and leaking, one can see the entire abnormal vasculature in the OCTA image, while it may be blocked by hyperfluorescence on a fluorescein angiogram. The ability to view impaired perfusion is another valuable component of OCTA, as non-moving blood will appear black on the image, much like FA.
All about layers
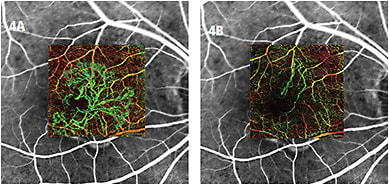
The OCTA systems allow for visualization of separate layers, or as a composite image of all the layers. Composite images can be a bit confusing for those who are used to viewing two-dimensional fluorescein or ICG angiograms. The composite OCT angiogram illustrates vascular flow from the superficial retinal, deep retinal plexus, choriocapillaris and choroid in a single three-dimensional image. The ability to separate the vascular layers is helpful, especially when viewing abnormal blood vessels in a layer that is typically avascular. OCT angiography software also assigns pseudocolor to vessels, depending on their depth in the tissue, which helps the viewer appreciate in which level the vessels present (Figures 3, 4).

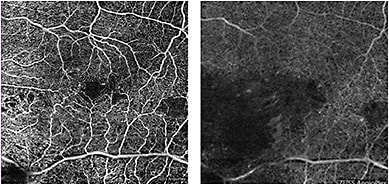
Another crucial difference is that deep retinal plexus vasculature is visible on OCTA but obscured in standard FA. The ability to visualize vascular flow in the deep retina plexus may expand knowledge of the physiology and treatment of retinal disease, as the effect of some disease processes on the deep retinal plexus is more extensive than the superficial retinal vessels, which is what the vascular layer imaged on FA (Figure 5).
You cannot ignore the flow B-scan
Commercially available OCTA systems offer a line B-scan with pseudocolor “flow.” This is not a measurement of rate of flow, merely an indication of the presence of movement. The flow B-scan is important to help identify vessels that present outside the normal layers, such as retinal angiomatous proliferation (RAP) lesions and CNV. The ability to detect the presence of vessels in this format also allows for differentiating between artifact and true blood movement.
Diagnose and track disease
OCTA can be helpful in identifying the following diseases:
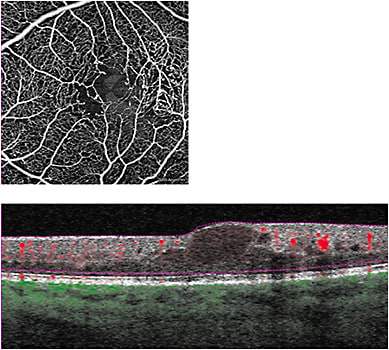
- Macular telangiectasia (MacTel). MacTel is defined as dilated parafoveal capillaries, microaneurysms, and, in some cases, subretinal neovascularization. On standard OCT, MacTel presents as intraretinal edema. FA reveals microaneurysms and capillary nonperfusion and has long been the gold standard for identifying MacTel. With OCTA, capillary insufficiency around the fovea and microaneurysms are evident. With the ability to view multiple layers not evident on FA, the extent of MacTel can be more defined on OCTA (Figure 6).
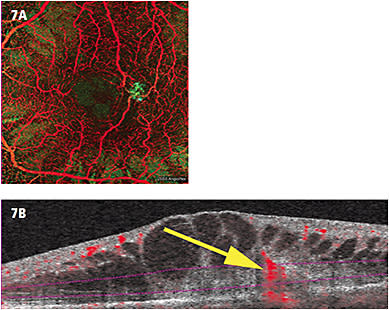
- RAP. RAP is a subset of exudative macular degeneration that is defined as a neovascular membrane that has a retinal vascular source, versus typical exudative AMD that has a choroidal vascular source. Again, with the advantage of en face imaging, viewing multiple layers of the intraretinal, subretinal and choroidal on OCTA to identify a retinal vascular abnormality is a clear advantage (Figure 7).
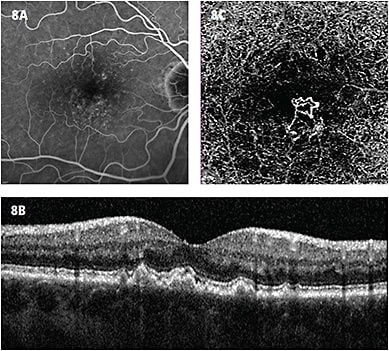
- Non-exudative choroidal neovascularization (CNV). To establish what “normal” looked like on OCTA, we had to scan every patient, regardless of the disease. In doing so, we noted that in approximately 10% of patients with “dry” AMD (defined by the presence of drusen and/or history of exudative AMD in other eye), there was a neovascular lesion at the sub-RPE level that was not evident on standard OCT or dye angiography.2 We defined this as “non-exudative CNV” (Figure 8) and are presently following the natural course of this finding.
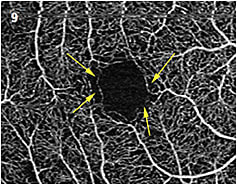
- Early changes in diabetic retinopathy. Patients with diabetes are advised to have a dilated eye exam on an annual basis. Without any evidence of hemorrhage or edema, these patients typically aren’t evaluated with angiography. OCTA may be useful to identify early changes in the parafoveal capillaries before typical changes are seen (Figure 9). These smaller capillaries may be some of the first to have impaired perfusion and can be part of a loss of the greater capillary bed. It is prudent to state that we have yet to establish a normative parafoveal capillary database, so any abnormalities seen may be a normal variant. The only way to be sure if the vessels are damaged is to establish a baseline for the individual patient then follow up with OCTA to identify changes.
Conclusion
It will take some time to create protocols for scanning, and those will vary by practice. As was the case in the early days of OCT, pathology presents itself differently than has been previously imaged. It is essential to identify what is normal and to establish a normative framework so that we can then identify what is abnormal.
We are witness to a revolution in ophthalmic imaging, and it is important for imagers and technicians to network with each other as we forge our way forward to develop nomenclature and standards that will become the benchmarks. OP
REFERENCES:
- Landry DA. Optical Coherence Tomography: A Clinical Atlas of Retinal Images 2011: Bryson Taylor Publishing.
- Landry DA, Kashani AH. OCT and OCT Angiography: Clinical Reference and Case Studies 2016: Bryson Taylor Publishing.









